publications
publications in reversed chronological order.
2026
- Transition from classical to ultimate meltingEdoardo Bellincioni, Kevin Zhong, Christopher J. Howland, and 4 more authorsarXiv preprint, 2026
Melting is omnipresent in nature and technology, with applications ranging from metallurgy, biology, food science, and latent thermal energy storage to oceanography, geophysics, and climate science, and occurring on all scales from sub-millimeter to global scales. The key objective is to understand the rate at which an object melts as a function of its size and of the ambient conditions. To achieve this it is important to be able to extrapolate from small scale experiments and observations to large or even global scales. This is done by scaling laws. However, these are only meaningful if there is no transition from one scaling relation to another one. Here we show, however, that for both fixed and freely-advected melting objects immersed in a turbulent flow a melting transition does exist, namely from slow melting at the small scales to fast melting at the large scales. We do so by controlled melting experiments and corresponding direct numerical simulations, covering four orders of magnitude in scale. The transition corresponds to the transition from a laminar-type boundary layer around the melting object to a turbulent-type boundary layer, i.e., from so-called classical turbulence to ultimate turbulence, with its enhanced transport properties. Our results thus provide a quantitative understanding of the flow physics of the melting process and thereby enable a better extrapolation and prediction of melt rates on large scales such as relevant in geophysics, oceanography, and climate science.
@article{bellincioni2026transitionclassicalultimatemelting, title = {Transition from classical to ultimate melting}, author = {Bellincioni, Edoardo and Zhong, Kevin and Howland, Christopher J. and Zhou, Yiyu and Huisman, Sander G. and Verzicco, Roberto and Lohse, Detlef}, year = {2026}, eprint = {2601.06517}, archiveprefix = {arXiv}, primaryclass = {physics.flu-dyn}, journal = {arXiv preprint}, doi = {10.48550/arXiv.2601.06517}, url = {https://arxiv.org/abs/2601.06517} }
2025
-
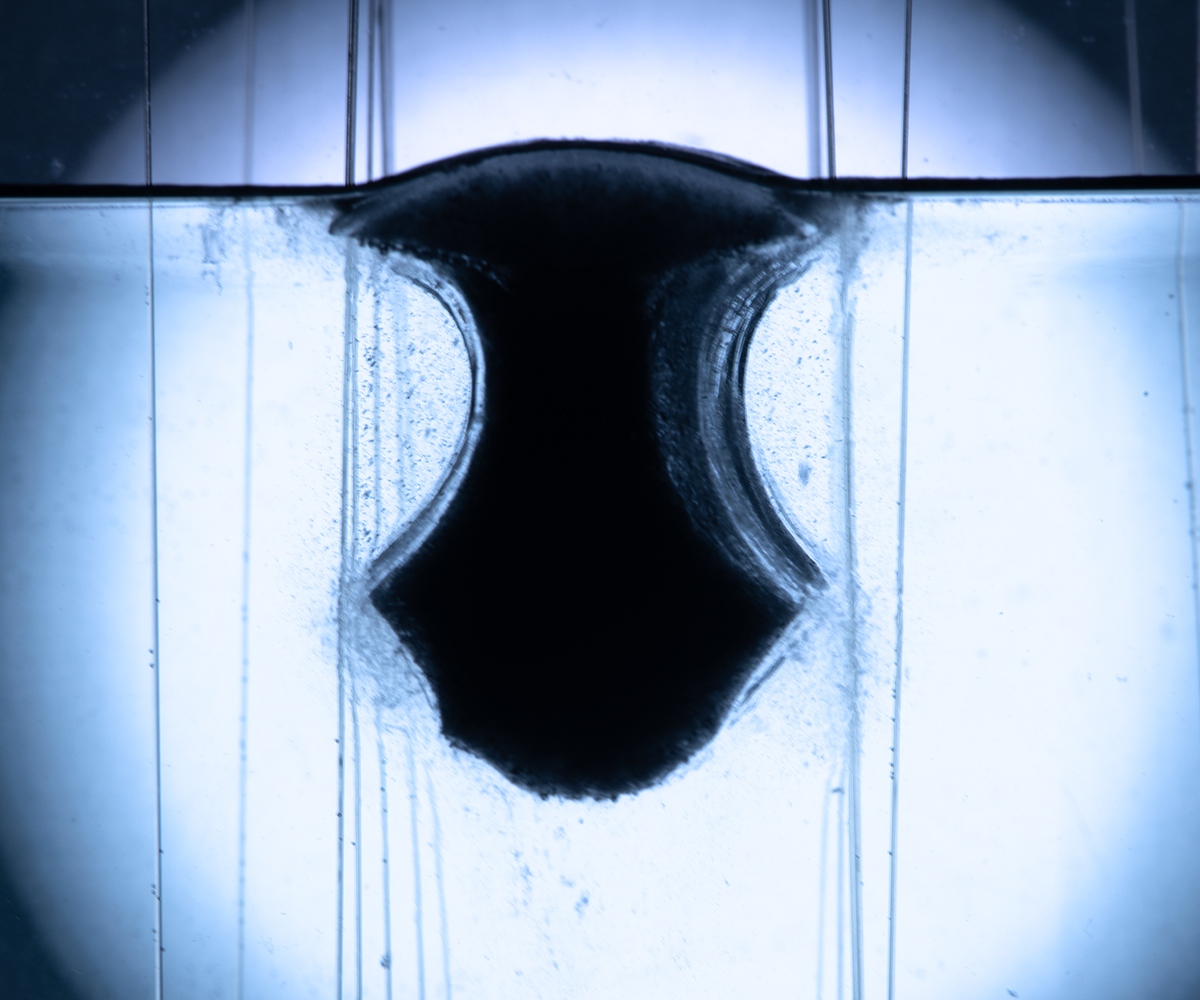 Melting of floating ice cylinders in fresh and saline environmentsEdoardo Bellincioni, Detlef Lohse, and Sander G. HuismanJournal of Fluid Mechanics, 2025
Melting of floating ice cylinders in fresh and saline environmentsEdoardo Bellincioni, Detlef Lohse, and Sander G. HuismanJournal of Fluid Mechanics, 2025Motivated by the need for a better understanding of the melting and stability of floating ice bodies, we experimentally investigated the melting of floating ice cylinders. Experiments were carried out in a tank, with ice cylinders with radii between 5 and 12 cm, floating horizontally with their axis perpendicular to gravity. The water in the tank was at room temperature, with salinities ranging from 0 to 35 g/L. These conditions correspond to Rayleigh numbers in the range 10^5 Ra 10^9. The relative density and thus the floating behaviour was varied by employing ice made of H2O–D2O mixtures. In addition, we explored a two-layer stable stratification. We studied the morphological evolution of the cross-section of the cylinders and interpreted our observations in the context of their interaction with the convective flow. The cylinders only capsize in fresh water but not when the ambient is saline. This behaviour can be explained by the balance between the torques exerted by buoyancy and drag, which change as the cylinder melts and rotates. We modelled the oscillatory motion of the cylinders after a capsize as a damped nonlinear oscillator. The downward plume of the ice cylinders follows the expected scalings for a line-source plume. The plume’s Reynolds number scales with Rayleigh number in two regimes, namely Re ∝ Ra^1/2 for Ra < O(10^7) and Re ∝ Ra^1/3 for Ra > O(10^7), and the heat transfer (non-dimensional as Nusselt number) scales as Nu ∝ Ra^1/3. Although the addition of salt substantially alters the solutal, thermal and momentum boundary layers, these scaling relations hold irrespectively of the initial size or the water salinity. While important differences exist between our experiments and real icebergs, our results can qualitatively be connected to natural phenomena occurring in fjords and around isolated icebergs, especially with regard to the melting and capsizing behaviour in stratified waters.
@article{Bellincioni_Lohse_Huisman_2025, title = {Melting of floating ice cylinders in fresh and saline environments}, volume = {1019}, doi = {10.1017/jfm.2025.10598}, journal = {Journal of Fluid Mechanics}, author = {Bellincioni, Edoardo and Lohse, Detlef and Huisman, Sander G.}, year = {2025}, pages = {A29}, }
2021
-
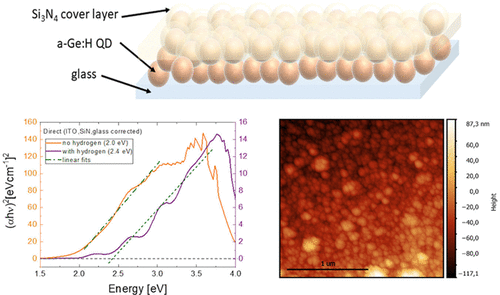 Inserting Hydrogen into Germanium Quantum DotsElisa Vitiello, Courtney H. Schreiber, Emma X. Riccardi, and 8 more authorsThe Journal of Physical Chemistry C, 2021
Inserting Hydrogen into Germanium Quantum DotsElisa Vitiello, Courtney H. Schreiber, Emma X. Riccardi, and 8 more authorsThe Journal of Physical Chemistry C, 2021Germanium quantum dots are very interesting for applications such as solar cells, photodetectors, and light emitters because their small bandgap can be tuned over a wide energy range by changing the particle size. One obstacle to applications is the presence of defects, both in the interior and at the surface of the nanoparticles. The defects function as nonradiative recombination centers or trap charge carriers, which will hinder further optical performance. Introducing hydrogen, as employed in a-Si:H solar cells, has proven to be a good method to counter such detrimental defect effects. In this work, germanium quantum dots were fabricated by an ultraclean, vacuum-based nanoparticle reactor in which hydrogen was supplied during growth. Optical spectroscopy of the a-Ge:H quantum dots, together with Raman and X-ray photoelectron spectroscopy, revealed a direct bandgap and that the presence of hydrogen resulted in amorphous Ge:H quantum dots. These a-Ge:H quantum dots are a step forward toward reducing charge carrier recombination in quantum dot solar cells.
@article{doi:10.1021/acs.jpcc.1c07019, author = {Vitiello, Elisa and Schreiber, Courtney H. and Riccardi, Emma X. and Nedell, Jack G. and Bellincioni, Edoardo and Parravicini, Jacopo and Binetti, Simona O. and Podestà, Alessandro and Lenardi, Cristina and Pezzoli, Fabio and Di Vece, Marcel}, title = {Inserting Hydrogen into Germanium Quantum Dots}, journal = {The Journal of Physical Chemistry C}, volume = {125}, number = {44}, pages = {24640-24647}, year = {2021}, doi = {10.1021/acs.jpcc.1c07019}, url = { https://doi.org/10.1021/acs.jpcc.1c07019}, eprint = {https://doi.org/10.1021/acs.jpcc.1c07019}, }